Process analysis using micro-discharge OES (µDOES) (Dipl.-Chem. Bastian Wiggershaus, Prof. Dr Carla Vogt)
Main component and trace analysis of industrial salt solutions
Both in the production of battery metals and in recycling, there is a growing need for fast, precise and easy-to-use analytical methods, especially for the on-line/on-site analysis of lithium salt solutions, the derived products of which are used in the battery industry. Typically used techniques such as ICP-OES or ICP-MS are usually limited to laboratory use, as they are not suitable for real-time analysis and monitoring of industrial processes on site due to high gas flows (Ar, He), high power consumption and sensitive spectrometer technology. Micro-discharge OES represents a promising alternative for this problem.
Principle of micro-discharge OES
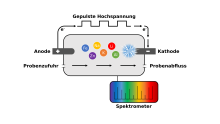
The method is based on optical emission spectroscopy, in which excited atoms and ions emit electromagnetic radiation of a characteristic wavelength. In contrast to conventional ICP-OES, the plasma is generated directly in the aqueous sample without the use of a carrier gas. For this purpose, the sample is fed into a measuring cell equipped with two rod electrodes (tungsten, glassy carbon). Using special high-voltage electronics, a high electrical power of up to 1-2 kW is channelled into a small volume of less than 1 mm3 around the cathode.
By applying high-voltage pulses to the electrodes, a microplasma is generated at the cathode, directly in the liquid sample. The microplasma is formed by a corona discharge and can be characterised as a non-equilibrium water vapour plasma. The light emitted by the excited atoms contained in the plasma region is transmitted to the spectrometer via a fibre optic cable, whereby spectra with atomic lines are predominantly obtained. During a single analytical measurement, typically 1000-3000 approx. 1 ms long microplasma pulses are generated and averaged. Depending on the application, various parameters such as the electrical conductivity of the sample, the energy of the plasma discharge, the electrode material or spectrometer settings can be adjusted.
Application
In cooperation with industrial partners, the process chain for extracting LiOH∙H2O in battery quality was analysed fully automatically. The first step is the leaching of a previously crushed and calcined lithium ore, whereby in addition to Li, other elements are present in sometimes significantly higher (Na, K) or lower concentrations (Ca, Mg, Rb). As the element contents are typically in the g/L range, the process samples are first diluted with ultrapure water and analysed after adjusting the conductivity. This real-time analysis enables fluctuations in the process to be identified, allowing process parameters to be quickly adjusted for optimum leaching efficiency.