Iron and SE compounds as reference materials for X-ray spectroscopy
Over the past two decades, synchrotron-based measurement techniques have established themselves as driving forces in areas such as materials and energy research to gain new insights into the composition, structure and function of different materials. These measurement techniques benefit from the unique characteristics of synchrotron radiation, such as polarisation, coherence and high brilliance. It was this brilliance in particular that enabled X-ray near-edge spectroscopy (XANES) to become established in the investigation of questions in areas ranging from materials research and geology to the life sciences. XANES spectroscopy utilises the fact that the electronic structure of the bonds is reflected in the spectrum of a substance and can therefore be used to determine the structure of substances or identify substances with the help of reference substances. The development of spectrometers for laboratory XANES is still being worked on today in order to be able to analyse a larger number of samples under routine conditions in line with better availability of the technology. One of the main technical challenges in this development of laboratory XANES is the low brilliance of the bremsstrahlung of X-ray tubes.
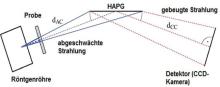
A laboratory XANES spectrometer at TU Berlin (AK Kanngießer), which uses a graphite mosaic crystal (HAPG) in von Hamos geometry [1], is being used to investigate optimised procedures for sample preparation and quantification models for spectrum evaluation, with a focus on iron and rare earth compounds. The aim is to validate this method for practical applications, for which the synthesis and characterisation of suitable reference materials is necessary. With the help of these standards, the influence of sample parameters such as particle size, mass occupancy, homogeneity and matrix composition on the spectra and the evaluation of the same are investigated, whereby various mathematical models for the evaluation of the spectra are tested. Initial results for these systematic investigations were obtained for the model system iron, for which compounds with iron in different oxidation states and with different binding partners were investigated (oxides, hydroxides, sulphides, silicates, iron complexes) in order to define the advantages and limits of the device setup.
Cooperation partners
Prof. B. Kanngießer, Dr W. Malzer, TU Berlin