Tetrahedral and octahedral silicon coordination in silicophosphates
Silicon is one of the most common elements on Earth. In most natural minerals, it is tetrahedrally surrounded by oxygen. However, there are also minerals in which silicon is sixfold coordinated. In most of these compounds, it is surrounded by phosphate groups. These silicophosphates have interesting material properties. Their high refractive index makes them interesting for optical applications and they are also used as catalysts.
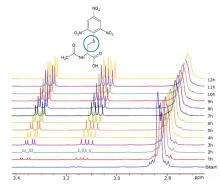
In cooperation with Prof Kroke's working group at the Institute of Inorganic Chemistry (Silicon Chemistry and Chemical Materials Science Group), new syntheses for silicophosphates are being developed [1-3]. For structural characterisation, mainly 1D and 2D solution and solid-state NMR methods are used, such as cross-polarisation (CP) and direct excitation (SP) measurements, heteronuclear correlation experiments and relaxation time measurements.
In combination with quantum chemical calculations performed by AK Prof. P. Kroll, Department of Chemistry and Biochemistry at the University of Arlington/ Texas, 29Si and 31P NMR shift tensors will be calculated and the driving forces and reaction conditions supporting the formation of sixfold coordinated silicon will be investigated.
Publications
- [1] Sandra Jähnigen, Erica Brendler, Uwe Böhme, Edwin Kroke; Silicophosphates containing SiO6 octahedra obtained from tetraalkoxysilanes, H3PO4 and P4O10 at ambient condition, New J. Chem. 38, 2014, 744-51; DOI: 10.1039/c3nj00721a
- [2] Sandra Jähnigen, Erica Brendler, Uwe Böhme, Edwin Kroke; Synthesis of silicophosphates containing SiO6-octahedra under ambient conditions - reactions of anhydrous H3PO4 with alkoxysilanes, Chem. Commun. 48, 2012, 7675-7677; DOI: 10.1039/C2CC31600E 2012; Cover
- [3] Sandra Jähnigen, Uwe Böhme, Gerhard Heide, Erica Brendler, Edwin Kroke; "Silicate-phosphate materials (SiOPS)"; DE 10 2012 011 034 A1 2013.12.05
BMBF joint project "ProHapTox"
Development of a reactivity-based test strategy for the animal-free detection of the skin sensitisation potential of electrophilic and pro-electrophilic industrial chemicals within the framework of the REACH chemicals regulation
The most important step in skin sensitisation is the reaction of small molecules (haptens) with peptides and proteins. The resulting products are recognised by the immune system, leading to the development of contact allergies. The Local Lymph Node Assay (LLNA) is currently used to analyse haptens[1]. This method requires a large number of test animals. It is therefore of great interest to develop alternative methods for investigating the skin sensitisation potential of chemicals. Our work focusses on the investigation of the reaction of haptens with peptides and proteins. Both NMR spectroscopy and mass spectrometry are used for this purpose. NMR can be used to determine the reactive centre and the reaction mechanism. Rate constants and activation energies are determined by reaction tracking and temperature-dependent analyses. Mass spectrometry is mainly used to differentiate between product mixtures and multiple adducts. However, HPLC chemoassay is often preferred here due to the low solubility of some substances[2]
.
Partners:
- Institute of Organic Chemistry, TU Bergakademie Freiberg
- Department of Ecological Chemistry, UFZ Leipzig
Sources:
- [1] Kimber I., Basketter D.A., FD Chem Toxic. 1992, 30,165-169.
- [2] Böhme A. et al., Chem. Res. Toxicol. 2009, 22, 742-750.