A variety of modern materials are used today as implant materials with support functions in the organism. These primarily include steels, titanium alloys and titanium, ceramic materials and polymers. The interactions with tissue and body fluids that take place on the surface of the implants require investigation because in unfavourable cases they can lead to limited applicability of the implant, its degradation or undesirable body reactions. In the development of modern implant materials, biocompatible materials that dissolve in the organism and coatings for permanent implants that release active substances have been current research priorities for some time.
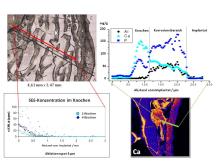
Resorbable implants for use in the musculoskeletal system should ideally perform support functions for a necessary period of time, but then be removed from the organism through biological degradation processes, making a second operation unnecessary. Mg alloys are well suited for this purpose as they have similar elasticity moduli to bone tissue and are highly biocompatible. Other alloy formers, such as Zn, Li and rare earths, are transported, deposited and excreted in the organism when the alloy dissolves. In order to test the biocompatibility of these new alloys and clarify the degradation mechanism,
- surface properties and element distributions in the alloys were investigated before implantation
- the concentration of the elements contained in the alloys in the surrounding bone material, the most important organs and in blood and urine (determination of background values)
- the concentration of these elements was determined in all important organs and fluids after different residence times of the implants in the animal organism
- investigations were carried out to clarify the degradation mechanism of the alloy used as an implant in the organism and to provide explanations for the observed growth process during the formation of new bone.
The aim of applying coatings to implants is to ensure that they heal better after surgery. Targeted drug release from these coatings can also serve to ensure the long-term function of the implant if, for example, anti-inflammatory agents or agents that inhibit cell division, as well as growth hormones, ensure optimal interaction between the implant surfaces and the surrounding tissue. The process of releasing the active ingredients can be controlled by the chemical and structural design of the coating. However, when developing or improving such coatings, it is necessary to control the release processes before clinical application. In various studies on the optimisation of coatings on stents, investigations were therefore carried out into the release of the active ingredients and the degradation of resorbable coatings. To this end,
- the degradation of polylactate coatings in the animal model was determined as a function of the implantation time
- and the degree of restenosis when different proliferation-inhibiting agents were applied.
Analysis methods used
| Analytical problem | Analysis methods used |
|---|---|
| Determination of the composition and homogeneity of the implant materials before and after implantation | REM-EDX, µ-XRF, ICP-OES, ICP-MS, PIXE, SY-XRF, IR, Raman |
| Element contents in bone, liver, blood, urine before and after implantation | ICP-MS, LA-ICP-MS, GF-AAS, PIXE, IC, ISE |
| Interaction alloy - proteins from bone (new bone formation) | SY-IR |
| Degradation mechanism | µ-RFA, PIXE, SY-RFA |
| Restenosis depending on the active substance in the coating | µ-RFA, PIXE, µ-Raman |
Cooperation partners
- Prof. F. Witte, Charité Berlin
- PD Dr J. Reifenrath, MHH