Current topics of the Mazik group
- Selective ligands for strategic elements
- Supramolecular motifs in the crystalline state
- Hydrogen bond patterns and water clusters
- Halogen bonds
- Development of artificial carbohydrate receptors
- Ion pair recognition with artificial systems
- Development of systems for applications in chemical sensor technology
- Conformation analysis

Selective ligands for strategic elements
Extraction of strategic and critical elements from the fly ash of (waste) incineration plants
The strategic elements indium (In) and gallium (Ga) as well as the base metal tin (Sn), are of great importance for numerous industrial applications (including communication technology, photovoltaics, electromobility). However, they are also considered to be critical raw materials due to their highly limited availability. For this reason, our research project is focussing on the effective and selective recycling of these raw materials.
For recycling, solvent extraction processes are used, which are based on an acidic, aqueous solution of the corresponding metal salts. The latter are transport back into an aqueous environment with the help of suitable ligand molecules via an organic solvent phase.

Selective extraction of indium ions from acidic aqueous solutions
M. M. Schulze, R. Löwe, R. Pollex, M. Mazik, Monatsh. Chem. 2019, 150, 983-990.
"Structure-extractability relationships for substituted 8-hydroxyquinolines: solvent extraction of indium ions from acidic aqueous media"
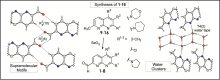
Hydrogen bond patterns and water clusters
R. Rosin, W. Seichter, A. Schwarzer, M. Mazik, Eur. J. Org. Chem. 2017, 6038-6051.
"1,8-Naphthyridine carbaldehydes and their methyl-substituted precursors: Synthesis, molecular structures, supramolecular motifs and trapped water clusters"
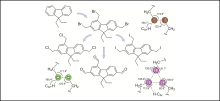
Halogen bonds
P. Seidel, A. Schwarzer, M. Mazik, Eur. J. Org. Chem. 2019, 1493-1502.
"Fluorene Derivatives Bearing Halogenomethyl Groups: Synthesis, Molecular Structures, and Halogen/Hydrogen Bonding Patterns in the Crystalline State"

Development of artificial carbohydrate receptors
F. Amrhein, J. Lippe, M. Mazik, Org. Biomol. Chem. 2016, 14, 10648-10659.
"Carbohydrate receptors combining both a macrocyclic building block and flexible side arms as recognition units: Binding properties of compounds with CH2OH groups as side arms"
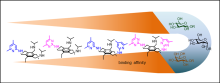
J. Lippe, W. Seichter, M. Mazik, Org. Biomol. Chem. 2015, 13, 11622-11632.
"Improved binding affinity and interesting selectivities of aminopyrimidine-bearing carbohydrate receptors in comparison with their aminopyridine analogues"

N. Koch, W. Seichter, M. Mazik, Synthesis 2016, 48, 2757-2767.
"Compounds based on a triethyl- or trimethoxybenzene scaffold bearing pyrazole, pyridine, and pyrimidine groups: Syntheses and representative binding studies towards carbohydrates"
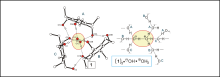
Ion pair detection with artificial systems
M. Stapf, W. Seichter, M. Mazik, Chem. Eur. J. 2015, 21, 6350-6354.
"Unique hydrogen-bonded complex of hydronium and hydroxide ions"
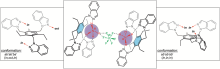
Development of systems for applications in chemical sensor technology
M. Schulze, N. Koch, W. Seichter, M. Mazik, Eur. J. Org. Chem. 2018, 4317-4330.
"Crystalline ammonium complexes of trimethyl- and triethylbenzene-based tripodal compounds bearing pyrazole and indazole groups"
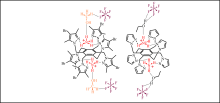
N. Koch, W. Seichter, M. Mazik, Tetrahedron 2015, 71, 8965-8974.
"Hexapodal pyrazole-based receptors: Complexes with ammonium ions and solvent molecules in the solid state"

Conformation analysis
M. Schulze, A. Schwarzer, M. Mazik, CrystEngComm 2017, 19, 4003-4016.
"Conformations of benzene-based tripodal isatin-bearing compounds in the crystalline state"