Investigation of (complex) hydrides as hydrogen storage systems using calorific methods
In the course of the global energy transition, i.e. the switch from fossil fuels to renewable energy sources, concepts for reversible energy storage are needed to compensate for fluctuations in the output of environmentally friendly energy sources that are only available intermittently. One variant currently being discussed is the chemical storage of hydrogen using complex hydrides such as NaAlH4:
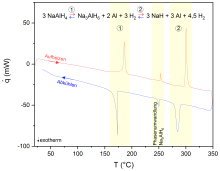
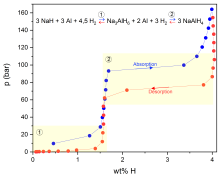
3 NaAlH4 ↔ Na3AlH6 + 2 Al + 3 H2 ↔ NaH + 3 Al + 4.5 H2
Hydrogen is to be stored by hydrogenating the decomposition products of the complex hydride and released again through its thermal decomposition.
A large number of these hydrides can be produced by metathesis reactions. The synthesis is carried out either in solution or mechanochemically in a ball mill.
The aim of research at the Institute of Physical Chemistry with regard to complex hydrides and other related compounds is to carry out fundamental investigations into their suitability and optimisation for reversible hydrogen storage. With regard to mobile applications, the development of hydrides for the high-pressure range is of particular interest. For this purpose, various complex hydrides and mixtures of these are thermodynamically characterised with regard to their dehydrogenation and hydrogenation behaviour. This is mainly done using thermogravimetry coupled with dynamic differential scanning calorimetry and decomposition gas analysis (TG-DSC-FTIR/MS), with DSC measurements under high hydrogen pressure (HP-DSC, see Fig. 1) and by recording pressure-composition isotherms with a Sieverts apparatus (see Fig. 2).
The investigation of complex hydrides using TG-DSC primarily serves to elucidate the dehydrogenation reactions.
The influence of the hydrogen pressure on the hydrogenation and dehydrogenation temperatures and the reversibility of the reactions as well as cycle stabilities can be studied on the basis of the HP-DSC measurements. Furthermore, statements and data on the thermodynamics and kinetics of the processes taking place can be derived.
The reversible hydrogen storage capacity and the equilibrium pressures of the hydrogenation-dehydrogenation cycles investigated at different temperatures can be determined by recording pressure-composition isotherms. This can also be used to determine the thermodynamic data of the reactions.
Topics for graduate theses:
For interested students, topics for student research projects, bachelor's and master's theses/dissertations can be arranged individually on an ongoing basis.
Selected publications:
Publications in journals:
F. Habermann, A. Wirth, K. Burkmann, B. Störr, J. Seidel, R. Gumeniuk, K. Bohmhammel, F. Mertens, „Heat Capacity Function, Enthalpy of Formation and Absolute Entropy of Mg(AlH4)2“, ChemPhysChem 2024, 25, doi.org/10.1002/cphc.202300748
F. Habermann, K. Burkmann, J. Kraus, B. Störr, J. Seidel, K. Bohmhammel, J. Kortus, R. Gumeniuk, F. Mertens, „Thermodynamic and kinetic Study of the thermal dehydrogenation of Sr(AlH4)2 taking into account the by-products NaCl and LiCl“, J. Alloys Compd. 2024, 980, 173476, doi.org/10.1016/j.jallcom.2024.173476
K. Burkmann, F. Habermann, E. Schumann, J. Kraus, B. Störr, H. Schmidt, E. Brendler, J. Seidel, K. Bohmhammel, J. Kortus, F. Mertens, „Structural and thermodynamic investigations of Zr(BH4)4 and Hf(BH4)4 between 280 K and their decomposition temperatures“, New J. Chem. 2024, 48, 2743–2754, doi.org/10.1039/D3NJ05601E
Z. Cao, F. Habermann, K. Burkmann, M. Felderhoff, F. Mertens, „Unstable Metal Hydrides for Possible On-Board Hydrogen Storage“, Hydrogen 2024, 5, 241–279, doi.org/10.3390/hydrogen5020015.
F. Habermann, K. Burkmann, B. Hansel, B. Störr, C. Schimpf, J. Seidel, M. Bertau, F. Mertens, „Hydrogen absorption and desorption in the V-Al-H system“, Dalton Trans. 2023, 52, 4880–4890, doi.org/10.1039/D2DT03718A
F. Taubert, D. Thomas, R. Hüttl, J. Seidel, F. Mertens, „Experimental determination of enthalpies of formation of Li17Si4, Li16.42Si4 and Li13Si4“, J. Alloys Compd. 2022, 897, 163147–163155, doi.org/10.1016/j.jallcom.2021.163147
L. Sandig-Predzymirska, J. Ortmeyer, J. Wagler, E. Brendler, F. Habermann, M. Anders, M. Felderhoff, F. Mertens, “The direct and reversible hydrogenation of activated aluminium supported by piperidine” Dalton Trans. 2020, 49, 17689-17698, doi.org/10.1039/D0DT03175E
J. Ortmeyer, A. Bodach, L. Sandig‐Predzymirska, B. Zibrowius, F. Mertens, M. Felderhoff, “Direct Hydrogenation of Aluminum via Stabilization with Triethylenediamine: A Mechanochemical Approach to Synthesize the Triethylenediamine ⋅ AlH3 Adduct”, Chemphyschem 2019, 20, 1360-1368, doi.org/10.1002/cphc.201801093
D. Thomas, N. Bette, F. Taubert, R. Hüttl, J. Seidel, F. Mertens, „Experimental determination oft he enthalpies of formation oft he lithium silicides Li7Si3 and Li12Si7 based on hydrogen sorption measurements“, J. Alloys Compd. 2017, 704, 398-405, doi.org/10.1016/j.jallcom.2017.02.010
Conference contributions:
Konrad Burkmann, Angus Demmer, Franziska Habermann, Jakob Kraus, Bianca Störr, Jürgen Seidel, Roman Gumeniuk, Jens Kortus, Klaus Bohmhammel, Florian Mertens, Investigation of the Potential for Hydrogen Storage in a Portfolio of Selected Boranates by Calorimetry, St. Malo, 2024
Franziska Habermann, Anneliese Wirth, Konrad Burkmann, Jakob Kraus, Bianca Störr, Jürgen Seidel, Jens Kortus, Roman Gumeniuk, Klaus Bohmhammel, Florian Mertens, Determination and Estimation of Thermodynamic Data of Complex Aluminum Hydrides, St. Malo, 2024
Angus Demmer, Konrad Burkmann, Bianca Störr, Roman Gumeniuk, Jürgen Seidel, Klaus Bohmhammel, Florian Mertens, Synthesis and Thermochemical Characterisation of Strontium Boranate, Gießen, 2024
F. Habermann, K. Burkmann, B. Störr, J. Seidel, K. Bohmhammel, F. Mertens, Study of the Influence of NaCl and LiCl on the Decomposition of Sr(AlH4)2, Die 25. Kalorimetrietage, Braunschweig 2023, pdf-Download
K. Burkmann, F. Habermann, J. Kraus, E. Schumann, B. Störr, J. Seidel, K. Bohmhammel, J. Kortus, F. Mertens, Thermodynamic Study of Zirconium and Hafnium Boranate – Zr(BH4)4 and Hf(BH4)4, Die 25. Kalorimetrietage, Braunschweig 2023, pdf-Download
Franziska Habermann, Bianca Störr, Jürgen Seidel, Florian Mertens, Thermodynamic Investigations on the Hydrogenation of Vanadium, Braunschweig/online, 2021
Franziska Habermann, Anneliese Wirth, Konrad Burkmann, Bianca Störr, Jürgen Seidel, Florian Mertens, Thermodynamic Investigations on the Dehydrogenation Reactions of the Complex Hydrides Mg(AlH4)2 and Ca(AlH4)2, Braunschweig/online, 2021
L. Sandig-Predzymirska, K. Burkmann, J. Seidel, F. Mertens, Influence of the aluminium addition on the hydrogenation/dehydrogenation behavior of doped sodium alanate Die 23. Kalorimetrietage, Braunschweig 2019, pdf-Download
Contact
- Prof. Dr. Florian Mertens (E-Mail: Florian [dot] Mertens [at] chemie [dot] tu-freiberg [dot] de (Florian[dot]Mertens[at]chemie[dot]tu-freiberg[dot]de))
- Dipl.-Chem. Konrad Burkmann (E-Mail: Konrad-Joerg [dot] Burkmann [at] chemie [dot] tu-freiberg [dot] de (Konrad-Joerg[dot]Burkmann[at]chemie[dot]tu-freiberg[dot]de))
- B. Sc. Angus Demmer (E-Mail: Angus [dot] Demmer [at] student [dot] tu-freiberg [dot] de (Angus[dot]Demmer[at]student[dot]tu-freiberg[dot]de))