Our working group focuses on material-related issues in the context of energy storage and conversion. The main topics are hydrogen storage, catalytic hydrogenation for energy storage and porous media. The material systems at the centre of our research are metal hydrides, transition metal catalysts and metal-organic frameworks (MOFs).
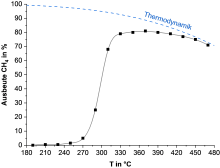
CO2 hydrogenation
In the "Gas phase catalysis" research focus area, the Institute of Physical Chemistry is primarily investigating the hydrogenation of carbon dioxide to methane. These investigations are based on the power-to-gas concept. In this concept, surplus energy from renewable sources is stored using water electrolysis. The resulting hydrogen is converted by carbon dioxide into methane, which is a very suitable energy storage medium for the natural gas network.
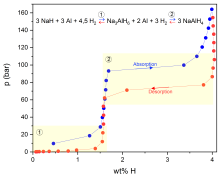
Investigation of (complex) hydrides as hydrogen storage using calorific methods
In the course of the global energy transition, i.e. the switch from fossil fuels to renewable energy sources, concepts for reversible energy storage are needed to compensate for fluctuations in the output of environmentally friendly energy sources that are only available intermittently. One variant currently under discussion is the chemical storage of hydrogen using complex hydrides such as NaAlH4.
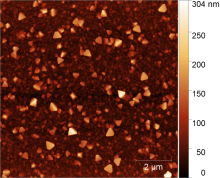
Metal Organic Frameworks - a class of crystalline, porous coordination polymers
One of the main areas of research at the Institute of Physical Chemistry is in the field of metal-organic coordination polymers, known as Metal Organic Frameworks (MOFs for short). The representatives of this class of materials are characterised by a bimodular structure consisting of inorganic nodes, so-called secondary building units (SBUs for short), and organic linker units coupled with the properties of permanent porosity and crystallinity. This makes it possible to imagine various possible applications in the fields of catalysis, gas storage, sensor technology and material separation.
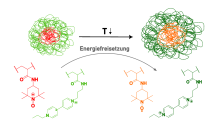
Redox-active thermoresponsive polymers for energy storage
The aim is to store energy in individual microgels that are based on core-shell structures and carry different electroactive groups in the various microgel domains. The core and shell consist of thermoresponsive polymers that collapse when the temperature rises and swell when the temperature drops below the transition temperature.
Visitor address
TU Bergakademie Freiberg
Faculty of Chemistry, Physics and Biosciences
Institute of Physical Chemistry
Lessingstraße 45
09599 Freiberg
Google Maps - Link

